2′-Deoxyuridine conjugated with a reactive monobenzocyclooctyne as a DNA building block for copper-free click-type postsynthetic modification of DNA†
Abstract
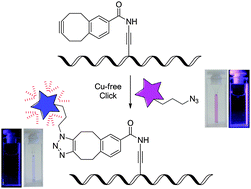
The carboxymethylmonobenzocyclooctyne group attached to the 5-position of a 2′-deoxyuridine in DNA allows rapid and efficient copper-free postsynthetic modification as demonstrated with a far-red emitting fluorescent azide probe. Upon labeling strong fluorescence intensity enhancement is observed.

 Please wait while we load your content...
Please wait while we load your content...