Chiral magnesium(ii)-catalyzed asymmetric ring-opening of meso-aziridines with primary alcohols†
Abstract
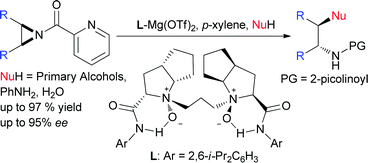
The asymmetric ring-opening of meso-aziridines with primary alcohols is realized using an N,N′-dioxide–Mg(OTf)2 complex as the catalyst. The desired vicinal trans-β-amino ethers are afforded in good yields and enantioselectivities. Aniline and water could also be used as the nucleophiles for the ring-opening in an identical catalyst system.

 Please wait while we load your content...
Please wait while we load your content...