Catalytic hydroxylation of benzene and toluene by an iron complex bearing a chelating di-pyridyl-di-NHC ligand†
Abstract
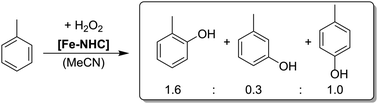
This work reports on iron-catalysed hydroxylation of benzene and toluene using aqueous H2O2. While benzene is hydroxylated with a high selectivity to phenol, toluene is hydroxylated to cresols with a high selectivity for the ortho and para-position. An inverse KIE indicates the presence of a high valent Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species during catalysis.
O species during catalysis.

 Please wait while we load your content...
Please wait while we load your content...