Catalytic asymmetric aminolactonization of 1,2-disubstituted alkenoic acid esters: Efficient construction of aminolactones with an all-carbon quaternary stereo-centre†‡
Abstract
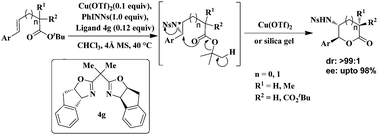
Chiral BOX–Cu(OTf)2 catalyzed enantioselective aminolactonization of the tert-butyl ester of alkenoic acids has been developed via in situ aziridination using PhINNs as the nitrene source. It provides exclusively trans-γ- and δ-amino lactones including an additional all-carbon quaternary stereo-centre with up to 98% ee in good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...