Unconserved substrate-binding sites direct the stereoselectivity of medium-chain alcohol dehydrogenase†
Abstract
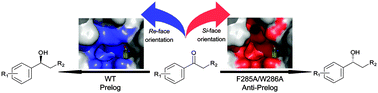
Structure-guided design of substrate-binding pocket inversed the stereoselectivity of an NADH-dependent medium-chain alcohol dehydrogenase (MDR) from Prelog to anti-Prelog. The pocket-forming amino acids, especially the unconserved residues as hotspots, play critical roles in directing MDRs' stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...