Highly diastereo- and enantioselective Michael addition of 3-substituted benzofuran-2(3H)-ones to 4-oxo-enoates catalyzed by lanthanide(iii) complexes†
Abstract
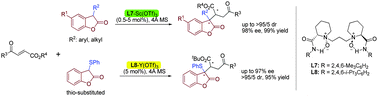
An efficient lanthanide(III)-catalyzed diastereo- and enantioselective Michael addition of 3-substituted benzofuran-2(3H)-ones to 4-oxo-enoates was developed. The desired adducts with contiguous quaternary–tertiary stereocenters were obtained in up to 99% yield with up to >95/5 dr and 98% ee.

 Please wait while we load your content...
Please wait while we load your content...