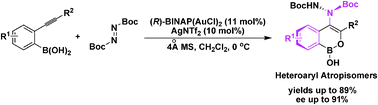
Asymmetric synthesis of heteroaryl atropisomers via a gold-catalyzed cycloisomerization–amination cascade reaction†
Abstract
The chiral gold(I) complex enables enantioselective cycloisomerization–amination of 2-(alkynyl)phenyl boronic acids and diazenes in high yields. A wide scope of substrates bearing various functional groups was tolerated to generate structurally different hydrazide derivatives as a new type of atropisomer.

 Please wait while we load your content...
Please wait while we load your content...