AgNO2-mediated direct nitration of the quinoxaline tertiary benzylic C–H bond and direct conversion of 2-methyl quinoxalines into related nitriles†
Abstract
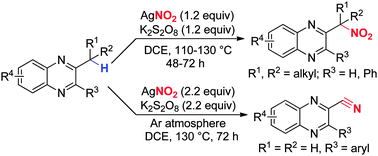
A unique method for AgNO2-mediated direct nitration of the quinoxaline tertiary C–H bond and direct conversion of 2-methyl quinoxalines into 2-quinoxaline nitriles under oxidative conditions has been developed. This protocol provides an efficient way to access quinoxaline containing nitroalkanes and nitriles depending on different substrate selection.

 Please wait while we load your content...
Please wait while we load your content...