Mechanism of the cysteine sulfenic acid O-sulfenylation of 1,3-cyclohexanedione†
Abstract
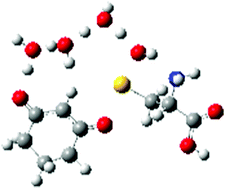
The density functionals B3LYP, B3PW91, M062X, and CAM-B3LYP with the 6-311+G(d,p) basis set predict the cysteine sulfenic acid O-sulfenylation of the s-cis-ketoenol tautomer of 1,3-cyclohexanedione proceeds through a cyclic 14-membered transition state structure containing three water molecules.

 Please wait while we load your content...
Please wait while we load your content...