Catalytic enantioselective nucleophilic addition of ynamides to aldehydes†
Abstract
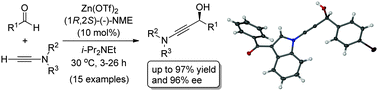
The first catalytic asymmetric addition of ynamides to aliphatic and aromatic aldehydes is described. This reaction provides unprecedented access to a diverse family of N-substituted propargylic alcohols that are obtained in high yield and ee in the presence of 10 mol% of zinc triflate and N-methylephedrine. The use of apolar solvent mixtures is essential to avoid product racemization and to optimize ee's without compromising conversion.

 Please wait while we load your content...
Please wait while we load your content...