Assembly of indenamine derivatives through in situ formed N-sulfonyliminium ion initiated cyclization†
Abstract
An expedient route to structurally diverse indenamine derivatives through condensation of the readily accessible substituted cinnamylaldehydes and sulfonylamines under the catalysis of FeCl3 has been developed, featuring high efficiency in the generation of two bonds and one ring in a single-step and water as the only by-product.
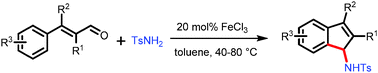

 Please wait while we load your content...
Please wait while we load your content...