Highly diastereoselective hydrosilylations of allylic alcohols†
Abstract
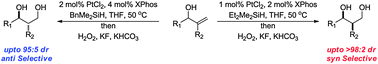
The highly syn-selective hydrosilylation of allylic alcohols was developed which, following oxidation, provided 1,3 alcohols containing two contiguous stereocentres. Through judicious choice of silane the complementary anti-selective hydrosilylation was also developed. This protocol was applied to the synthesis of an all syn polyketide stereotriad.

 Please wait while we load your content...
Please wait while we load your content...