Regioselective synthesis of oxazole derivatives via palladium-catalyzed and copper-mediated cascade oxidative cyclization†
Abstract
A novel Pd-catalyzed/Cu-mediated oxidative cyclization has been developed for the synthesis of trisubstituted oxazoles, which is thought to proceed through cascade formation of C–N and C–O bonds. In this protocol, four hydrogen atoms were removed and water was used as the oxygen atom source.
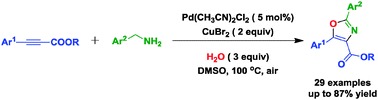

 Please wait while we load your content...
Please wait while we load your content...