Propargylic cation-induced intermolecular electrophilic addition–semipinacol rearrangement†
Abstract
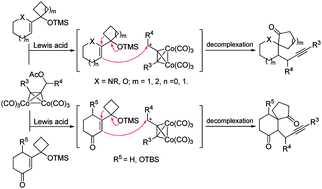
A novel propargylic electrophile-induced tandem intermolecular addition–semipinacol rearrangement was developed efficiently under mild conditions. Various allylic silylether substrates as well as Co-complexed propargylic species were applicable to this protocol and gave a series of synthetically useful β-propargyl spirocyclic ketones in moderate to good yields. Its synthetic application was also demonstrated by an efficient construction of the key tricyclic moiety of daphlongamine E.

 Please wait while we load your content...
Please wait while we load your content...