Iridium-catalyzed selective α-methylation of ketones with methanol†
Abstract
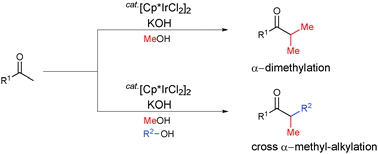
Iridium-catalyzed selective α-dimethylation and α-methylation of ketones or phenylacetonitriles, using methanol as the methylating agent, were achieved. In addition, three-component cross α-methyl-alkylation was successfully performed using methyl ketones with methanol and primary alcohols with long-chain alkyl groups. This method provides a very convenient direct route to α-methylated ketones, using methanol.

 Please wait while we load your content...
Please wait while we load your content...