Selective α-amination and α-acylation of esters and amides via dual reactivity of O-acylhydroxylamines toward zinc enolates†
Abstract
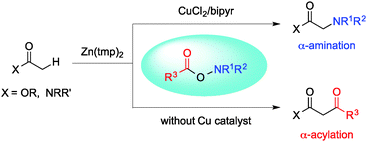
Selective α-amination and α-acylation of esters and amides have been developed, employing O-acylhydroxylamines as a dually reactive aminating and acylating reagent. Treatment of zinc enolates with O-acylhydroxylamines provides solely 1,3-dicarbonyl compounds under mild conditions. Introduction of a copper catalyst into the system shifts the reactivity entirely, yielding α-amination products exclusively.

 Please wait while we load your content...
Please wait while we load your content...