Novel regio- and stereoselective phosphonyl radical addition to glycals promoted by Mn(ii)–air: syntheses of 1,2-dideoxy 2-C-diphenylphosphinylglycopyranosides†
Abstract
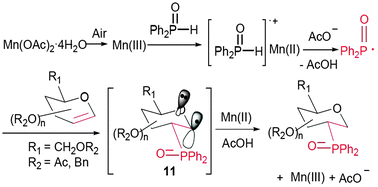
1,2-Dideoxy-2-C-diphenylphosphinylglycopyranosides were first synthesized by the novel Mn(II)–air promoted reaction of diphenylphosphine oxide with various glycals in high yields with excellent regio- and stereoselectivities, which was clarified as a radical addition reaction controlled by the oxygen of vinyl ether.

 Please wait while we load your content...
Please wait while we load your content...