Engineering entanglement: controlling the formation of polycatenanes and polyrotaxanes using π interactions†
Abstract
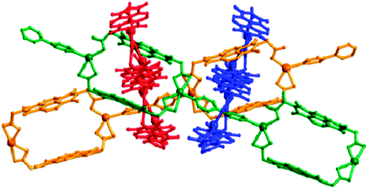
A reproducible metallocyclic motif containing amino-acid functionalised aromatic diimides has been employed to demonstrate remarkable control over entanglement topologies. [2]-Catenane and pseudo-rotaxane units give rise to 1D → 2D polycatenation, the formation of which can be sterically prevented, and a unique 1D → 3D polyrotaxane.

 Please wait while we load your content...
Please wait while we load your content...