Regioselective functionalization of 2-arylazetidines: evaluating the ortho-directing ability of the azetidinyl ring and the α-directing ability of the N-substituent†‡
Abstract
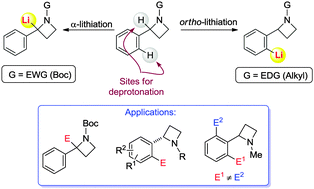
The regioselective lithiation–functionalization of 2-arylazetidines has been explored. The nature of the N-substituent is mainly responsible for a regioselectivity switch. ortho-Lithiation occurred, using hexyllithium as a greener base, in N-alkylazetidines, while α-benzylic lithiation has been observed with N-Boc azetidines.

 Please wait while we load your content...
Please wait while we load your content...