Self-promoted post-synthetic modification of metal–ligand M2L3 mesocates†
Abstract
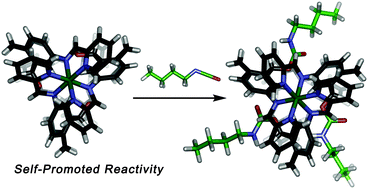
Reactive alcohol functionality has been incorporated into a self-assembled M2L3 mesocate. Post-synthetic modification of this complex with suitable isocyanates is not only possible, but is self-catalyzed by multiple internal hydrogen bonds from the self-assembly. As the metal–ligand coordination is reversible at elevated temperature, the isomeric distribution of product changes upon reaction, due to the different steric bulk conferred on the assembly after the modification.

 Please wait while we load your content...
Please wait while we load your content...