Dramatic reduction in the activation barrier for dinitrogen splitting using amine–borane as a hydrogen carrier: insights from the DFT study†
Abstract
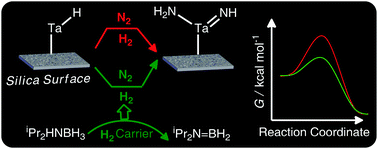
DFT based mechanistic investigations on a silica surface supported Tantalum system reveal that there is a significant reduction in the free energy activation barrier for N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond dissociation upon using a suitable amine–borane as a hydrogenating agent as compared to that of using molecular H2 for the same purpose, which suggests that dinitrogen dissociation can be achieved in surface chemistry at much lower temperatures than those in the presently used systems.
N bond dissociation upon using a suitable amine–borane as a hydrogenating agent as compared to that of using molecular H2 for the same purpose, which suggests that dinitrogen dissociation can be achieved in surface chemistry at much lower temperatures than those in the presently used systems.

 Please wait while we load your content...
Please wait while we load your content...