Highly diastereoselective synthesis of 3-hydroxy-2,2,3-trisubstituted indolines via intramolecular trapping of ammonium ylides with ketones†
Abstract
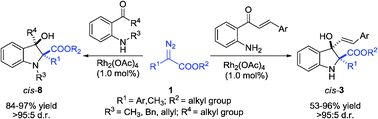
A Rh2(OAc)4-catalyzed diazo decomposition reaction of diazo esters with 2-aminophenyl ketones is reported. A series of 3-hydroxy-2,2,3-trisubstituted indolines are produced in good yields with excellent diastereoselectivities via an intramolecular aldol-type trapping of ammonium ylides with ketone units.

 Please wait while we load your content...
Please wait while we load your content...