Applications of pentafluorophenyl boron reagents in the synthesis of heterocyclic and aromatic compounds
Abstract
Recently, main group reagents have attracted a lot of attention in bond-forming reactions in organic synthesis. This article highlights the use of pentafluorophenyl substituted boron reagents in their reactions with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
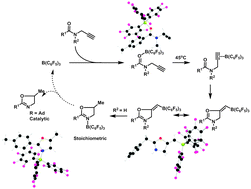
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C π-bonds for the synthesis of heterocyclic and aromatic compounds. These cyclisation reactions fall into four general classes although there is some overlap between classes and often combinations of these different types of reactivity are observed in the formation of the final heterocyclic product: (i) 1,2- (and 1,4-) additions of nucleophile and Lewis-acidic boron centre, (ii) 1,1-carboboration, (iii) carbocation rearrangements and (iv) cycloaddition chemistry. In addition, the prospect of using such boron reagents catalytically in the synthesis of aromatic compounds such as oxazoles and dibenzopentalene derivatives is emphasised.
C π-bonds for the synthesis of heterocyclic and aromatic compounds. These cyclisation reactions fall into four general classes although there is some overlap between classes and often combinations of these different types of reactivity are observed in the formation of the final heterocyclic product: (i) 1,2- (and 1,4-) additions of nucleophile and Lewis-acidic boron centre, (ii) 1,1-carboboration, (iii) carbocation rearrangements and (iv) cycloaddition chemistry. In addition, the prospect of using such boron reagents catalytically in the synthesis of aromatic compounds such as oxazoles and dibenzopentalene derivatives is emphasised.

 Please wait while we load your content...
Please wait while we load your content...