Enhanced oxidative stability of non-Grignard magnesium electrolytes through ligand modification†
Abstract
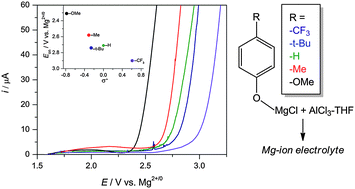
A series of non-Grignard Mg-electrolytes with various para-substituents was synthesized starting from commercially-available phenols. More electron-withdrawing substituents shift the anodic stability of the electrolyte by 400 mV. The p-CF3 substituted phenol exhibits the highest stability of 2.9 V vs. Mg2+/0, and cycles reversibly with the Chevrel-phase Mo6S8 Mg-ion cathode.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...