The first one-pot synthesis of a chiral pentakis-adduct of C60 utilising an opened-structure malonate tether†
Abstract
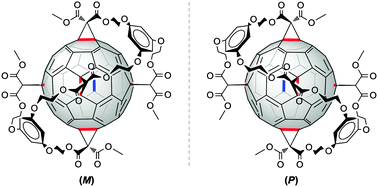
A pentakis-adduct of C60 with an incomplete octahedral addition pattern was synthesised via a one-pot procedure using a tether equipped with five malonate moieties. The five-fold Bingel cyclopropanation of C60 was completely regioselective and afforded a chiral, C2-symmetric pentakis-adduct which was easily separated by column chromatography on SiO2.

 Please wait while we load your content...
Please wait while we load your content...