One step synthesis of benzoxazepine derivatives via a PPh3 catalyzed aza-MBH domino reaction between salicyl N-tosylimines and allenoates†
Abstract
PPh3-Catalyzed aza-MBH domino reaction of salicyl N-tosylimines with γ-CH3 substituted allenoates is reported. Readily available imines and allenoates are converted to benzoxazepine derivatives in one step. Wherein, functionalization of C–H bonds of γ-substituted allenoate has been developed in this domino process.
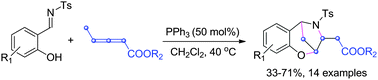

 Please wait while we load your content...
Please wait while we load your content...