Bicyclic guanidinium-catalyzed enantioselective phase-transfer alkylation: direct access to pyrroloindolines and furoindolines†
Abstract
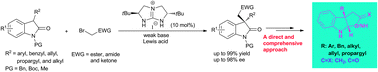
Highly enantioselective phase-transfer alkylation of 3-substituted-2-oxindoles with activated bromomethanes is disclosed with a broad substrate scope by using bicyclic guanidinium as a catalyst and a Lewis acid as the co-catalyst. The alkylation adducts are versatile intermediates to accomplish the syntheses of pyrroloindolines and furoindolines.

 Please wait while we load your content...
Please wait while we load your content...