Introduction of hydrophilic groups onto the ortho-position of benzoate anions induced phase separation of the corresponding ionic liquids with water†
Abstract
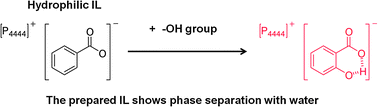
Ionic liquids (ILs) composed of tetrabutylphosphonium cations and benzoate anion derivatives with hydrophilic hydroxyl or carboxyl groups introduced onto the ortho-position were found to be less hydrophilic and showed phase separation with water, whereas unmodified benzoate-type IL was freely miscible with water.

 Please wait while we load your content...
Please wait while we load your content...