Stereochemical lability of azatitanacyclopropanes: dynamic kinetic resolution in reductive cross-coupling reactions with allylic alcohols† ‡
Abstract
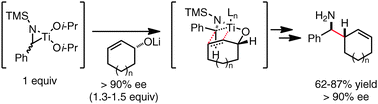
Azatitanacyclopropanes (titanaziridines) are shown to be stereochemically labile under reaction conditions for reductive cross-coupling. This fundamental property has been employed to realize highly selective asymmetric coupling reactions with allylic

 Please wait while we load your content...
Please wait while we load your content...