Palladium-catalyzed insertion of α,β-unsaturated N-tosylhydrazones and trapping with carbon nucleophiles†
Abstract
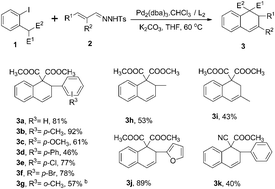
Palladium-catalyzed carbene migratory insertion–cyclization reactions were reported, delivering dihydronaphthalene and indene derivatives in moderate to good yields. A three-component cross-coupling was also developed. The reactions are easy to handle, under mild conditions and various functional groups are tolerated.

 Please wait while we load your content...
Please wait while we load your content...