A novel reaction of gem-difluorocyclopropyl ketones with nitriles leading to 2-fluoropyrroles†
Abstract
The CF3SO3H-promoted ring opening of gem-difluorocyclopropyl ketones prefers to undergo proximal bond cleavage and the subsequent cyclization with nitriles occurred smoothly to give 2-fluoropyrroles.
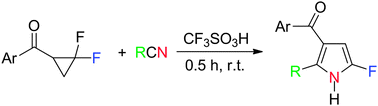

 Please wait while we load your content...
Please wait while we load your content...