The right way to self-fuse bi- and terpyrenyls to afford graphenic cutouts†
Abstract
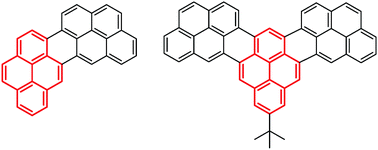
In this work, we subject bi- and terpyrenyls to selective fusion for formation of extended polycyclic aromatic hydrocarbons (PAHs). Connecting the pyrene units at 4-4′- or 1-4′-positions led to smooth formation of extended PAHs, achieved via cyclodehydrogenation. This is far more difficult if pyrene is coupled in the 1,1′-position.

 Please wait while we load your content...
Please wait while we load your content...