An efficient synthesis of polysubstituted pyrroles via copper-catalyzed coupling of oxime acetates with dialkyl acetylenedicarboxylates under aerobic conditions†
Abstract
A Cu-catalyzed [3+2]-type condensation reaction of oxime acetates and dialkyl acetylenedicarboxylates that provides highly substituted pyrroles under aerobic conditions is described. The newly formed pyrroles are easily employed for further transformations to prepare pyrrolo[2,1-a]isoquinoline skeletons.
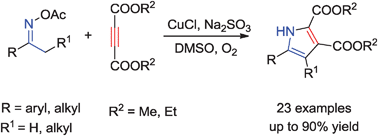

 Please wait while we load your content...
Please wait while we load your content...