Kinetic resolution of racemic α-hydroxyphosphonates by asymmetric esterification using achiral carboxylic acids with pivalic anhydride and a chiral acyl-transfer catalyst†
Abstract
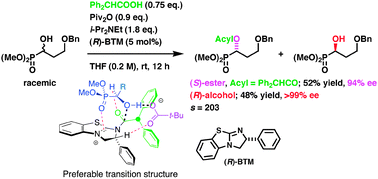
A practical protocol is developed to directly provide chiral α-acyloxyphosphonates and α-hydroxyphosphonates from (±)-α-hydroxyphosphonates utilizing the transacylation process to generate the mixed anhydrides from acid components and pivalic anhydride in the presence of organocatalysts (s-value = 33–518).

 Please wait while we load your content...
Please wait while we load your content...