Dibenzyl and diallyl 2,6-bisiminopyridinezinc(ii) complexes: selective alkyl migration to the pyridine ring leads to remarkably stable dihydropyridinates†
Abstract
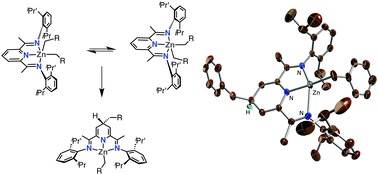
Diorganozinc compounds (ZnR2) with R = CH2Ph or CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 react with 2,6-bisiminopyridines (iPrBIP) to afford thermally stable dihydropyridinate(−1) complexes, and do not react if R = CH2SiMe3 or CH2CMe2Ph.
CH2 react with 2,6-bisiminopyridines (iPrBIP) to afford thermally stable dihydropyridinate(−1) complexes, and do not react if R = CH2SiMe3 or CH2CMe2Ph.

 Please wait while we load your content...
Please wait while we load your content...