Catalytic oxidative cleavage of catechol by a non-heme iron(iii) complex as a green route to dimethyl adipate†
Abstract
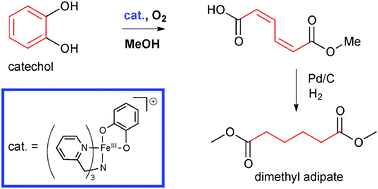
The catalyst system prepared in situ from iron(III) salts, tris(2-pyridylmethyl)amine and a base readily catalyses the intradiol dioxygenation of pyrocatechol in methanol, to primarily afford the half-methyl ester of muconic acid. Dimethyl adipate is obtained by the subsequent, one-step catalytic hydrogenation/esterification, thus providing a green route to this important nylon precursor.

 Please wait while we load your content...
Please wait while we load your content...