Asymmetric synthesis of gem-diaryl substituted cyclic sulfamidates and sulfamides by rhodium-catalyzed arylation of cyclic ketimines†
Abstract
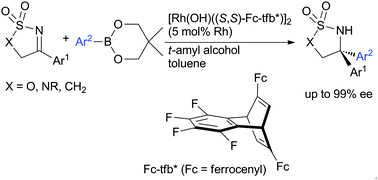
Asymmetric addition of arylboronates to aryl-substituted cyclic ketimines proceeded in the presence of a rhodium catalyst coordinated with a chiral diene ligand to give high yields of sulfamidates and sulfamides with high enantioselectivity (up to 99% ee).

 Please wait while we load your content...
Please wait while we load your content...