Anti-Bredt N-heterocyclic carbene: an efficient ligand for the gold(i)-catalyzed hydroamination of terminal alkynes with parent hydrazine†
Abstract
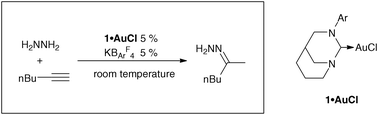
An anti-Bredt N-heterocyclic carbene gold(I) chloride complex was synthesized by taking advantage of the reversible insertion of the free

 Please wait while we load your content...
Please wait while we load your content...