Model studies towards the challenging angularly-oxygenated core of several angucyclinones from an oxidative dearomatization strategy†
Abstract
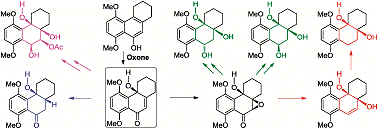
Up to six differently substituted highly challenging angularly-oxygenated tricyclic core models of natural angucyclinones were stereoselectively synthesized from a common p-quinol intermediate obtained from Oxone-mediated oxidative dearomatization of the corresponding tricyclic

 Please wait while we load your content...
Please wait while we load your content...