A new phenanthroline–oxazine ligand: synthesis, coordination chemistry and atypical DNA binding interaction†
Abstract
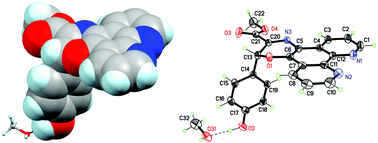
1,10-Phenanthroline-5,6-dione and L-tyrosine methyl ester react to form phenanthroline–oxazine (PDT) from which [Cu(PDT)2](ClO4)2 and [Ag(PDT)2]ClO4·2MeOH are obtained. Binding to calf-thymus DNA by Ag(I) and Cu(II) PDT complexes exceed bis-1,10-phenanthroline analogues and the minor groove binding drugs, pentamidine and netropsin. Furthermore, unlike the artificial metallonuclease, [Cu(phen)2]2+, the [Cu(PDT)2]2+ complex does not cleave DNA in the presence of added reductant indicating unique interaction with DNA.

 Please wait while we load your content...
Please wait while we load your content...