Synthesis of phenanthridinones viapalladium-catalyzed C(sp2)–H aminocarbonylation of unprotected o-arylanilines†
Abstract
An efficient synthesis of free (NH)-phenanthridinones through Pd-catalyzed C(sp2)–H aminocarbonylation of unprotected o-arylanilines under an atmospheric pressure of CO has been developed. Some ortho
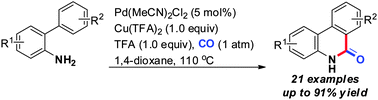

 Please wait while we load your content...
Please wait while we load your content...