Enantioselective synthesis of 2-substituted and 3-substituted piperidines through a bromoaminocyclization process†‡
Abstract
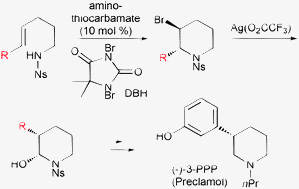
A catalytic enantioselective bromocyclization of olefinic amides using amino-thiocarbamates as the
- This article is part of the themed collection: Emerging Investigators 2013

 Please wait while we load your content...
Please wait while we load your content...