An efficient organocatalytic enantioselective Michael addition of aryl methyl ketones with 2-furanones: highly functionalized chiral 3,4-substituted lactones†
Abstract
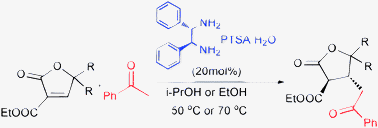
The efficient asymmetric Michael addition reactions of aryl methyl ketones with 2-furanones were catalyzed by a simple and commercially available chiral 1,2-diphenyl-1,2-ethanediamine and p-TSA·H2O as cocatalyst with good yields (up to 95%) and excellent enantioselectivities (up to >99% ee). A bi-functional catalytic mechanism for the reaction was proposed.

 Please wait while we load your content...
Please wait while we load your content...