Rhodium-catalyzed regio- and enantioselective amination of racemic secondary allylic trichloroacetimidates with N-methyl anilines†
Abstract
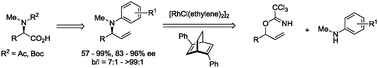
We report the chiral diene ligated rhodium-catalyzed dynamic kinetic asymmetric transformation (DYKAT) of racemic secondary allylic trichloroacetimidates with a variety of N-methyl

 Please wait while we load your content...
Please wait while we load your content...