Lewis acid-catalyzed unexpected selective C–C bond cleavage: an efficient and mild construction of cyclopentenes†
Abstract
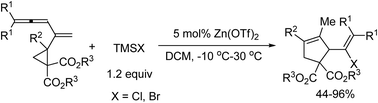
An intriguing Lewis acid-catalyzed intramolecular ring-opening/cyclization reaction of cyclopropylvinylallenes is developed. This process proceeds at ambient temperature to afford the structurally diverse cyclopentenes exclusively, which demonstrates the unique reactivity of 1,3,4-alkatrien-2-yl cyclopropanes.

 Please wait while we load your content...
Please wait while we load your content...