Copper-catalyzed ortho-acylation of phenols with aryl aldehydes and its application in one-step preparation of xanthones†
Abstract
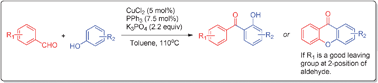
In the presence of triphenylphosphine, copper(II) chloride can catalyze an intermolecular ortho-acylation reaction of phenols with aryl aldehydes. The reaction proceeds smoothly with a wide range of starting materials, and furthermore, it can be used to synthesize xanthone derivatives in a single step in high yields.

 Please wait while we load your content...
Please wait while we load your content...