Novel transformation of α,β-unsaturated aldehydes and ketones into γ-amino alcohols or 1,3-oxazines via a 4 or 5 step, one-pot sequence†
Abstract
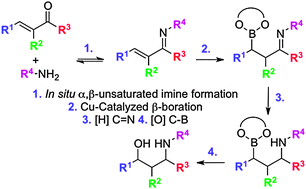
An efficient, 4-step, one-pot, highly stereoselective route to γ-amino alcohols has been developed via an in situ α,β-unsaturated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and
N) and

 Please wait while we load your content...
Please wait while we load your content...