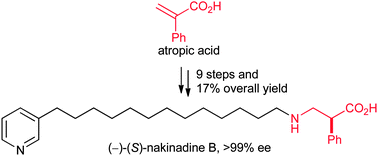
(−)-(S)-Nakinadine B: first asymmetric synthesis†
Abstract
(−)-(S)-Nakinadine B has been synthesized for the first time (in 9 steps and 17% overall yield from commercially available atropic acid) using the conjugate addition of lithium dibenzyl-amide to an N-α-phenylacryloyl SuperQuat derivative with in situ diastereoselective enolate protonation as the key step.

 Please wait while we load your content...
Please wait while we load your content...