An unusual conformational similarity of two peptide folds featuring sulfonamide and carboxamide on the backbone†
Abstract
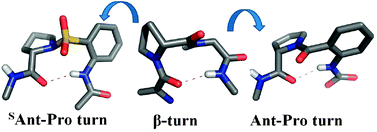
Two folded peptides featuring carboxamide and sulfonamide at the core of the peptide fold have been shown to possess almost similar conformational features, despite the well-known fact that carboxamides and sulfonamides have strikingly different hydrogen-bonding and geometrical preferences.

 Please wait while we load your content...
Please wait while we load your content...