Novel sulphur-rich BODIPY systems that enable stepwise fluorescent O-atom turn-on and H2O2 neuronal system probing†‡
Abstract
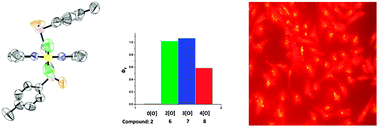
Bis-arylsulfide BODIPY systems were prepared and studied for multiple O-atom sensing (at 522 nm); 2- and 3-atom loading was optimal (50-fold, “turn on”). Neuronal studies showed greater H2O2 sensitivity than 2′,7′-dichlorofluorescein diacetate. The novel 1,3,6-trimethyl BODIPY formed as a biproduct under Lindsey conditions.

 Please wait while we load your content...
Please wait while we load your content...